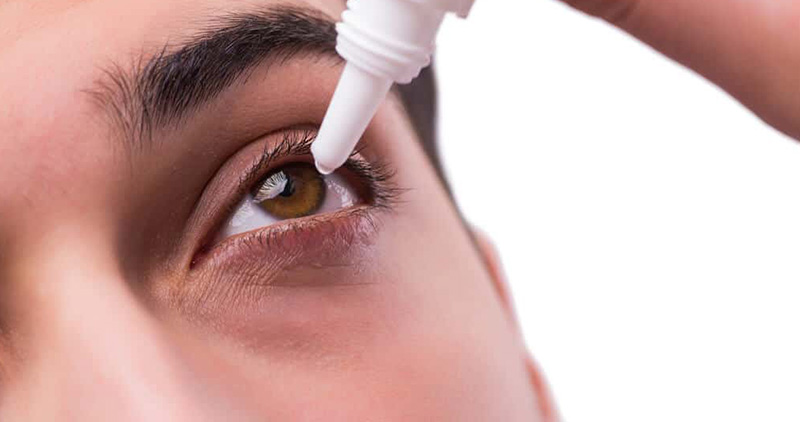
Eye Drop: Indian pharmaceutical company withdrew 27 eye drops from the US market, FDA had issued a warning of blindness
Mumbai-based Kilich Healthcare India Ltd recalled 27 types of eye drops sold in US stores. The US Food and Drug Administration (FDA) last month issued a warning to consumers not to buy the drugs sold by Walmart Inc., CVS Health Corporation, Target Corporation and other retailers.
The FDA had stated that these eyedrops could cause eye infection, visual impairment or blindness. However, the regulator did not name Kilich Healthcare India Limited while issuing the warning. According to a recent report by Bloomberg, Kilich made the eyedrops in an unsanitary factory in India, where some workers were working barefoot.
Violation of drug production rules
According to the report, FDA inspectors first visited Kilich’s factory in mid-October, where they found numerous violations of drug production regulations. One of them was that microbiologists were filling backdated test results in the records. Samples taken during the inspection also revealed bacterial contamination in the filling areas. The eyedrops were bottled at these places.